Cleaning Information
Correct reprocessing is fundamental in guarding against infection. To make liquid-based reprocessing more convenient, most BK Medical transducers come with a special watertight plug lid to allow the entire transducer (including the cable and plug) to be fully submerged.
BK Medical equipment is designed to be durable but as devices are complex, care is needed when handling them.
BK Medical continuously validates and evaluates different reprocessing agents and methods.
To download the care and cleaning guide with reprocessing posters, select your language
Care and Cleaning Methods for Transducers
Follow local regulations for minimum reprocessing. Check table 4 on page 29 of the Care and Cleaning Guide.
Follow product manufacturer’s instructions and do not exceed transducer specified limits. See Care and Cleaning Guide for more information.
Click the transducer to read the information.

I13C3f (9076) Advanced Laparoscopic Transducer
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
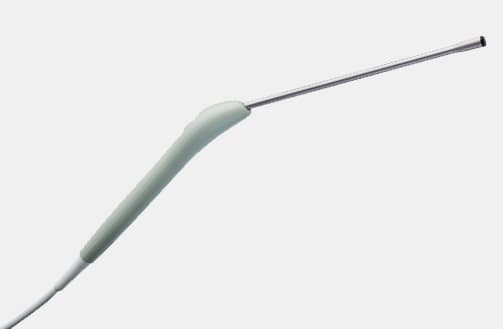
N20P6 (9007) Minimally Invasive Transducer

N20P6 (9007) Minimally Invasive Transducer d Footnote: d) Transducer has not been licensed by Health Canada.
 Print Cleaning Methods
Print Cleaning Methods
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.

Rob12C4 (9096) Robotic Transducer
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.

I12C5 (9034) Mini-T Intraoperative Transducer
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
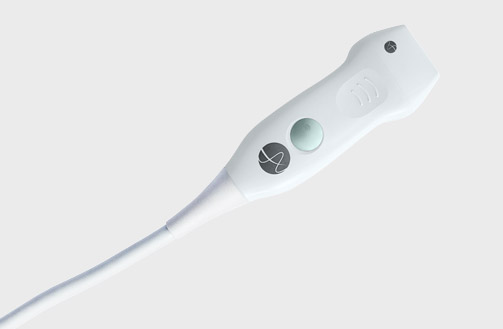
5P1e (9087) Phased Array Transducer

5P1e (9087) Phased Array Transducer b Footnote: b) Transducer connector is not immersible.
 Print Cleaning Methods
Print Cleaning Methods
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.

X18L5s (9009) Hockey Stick Transducer
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
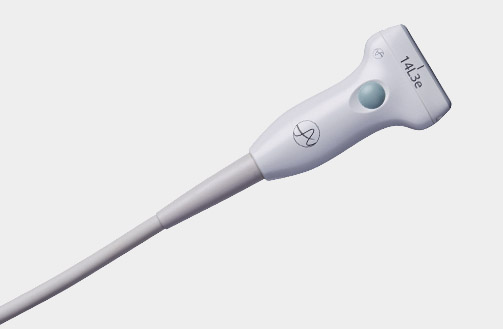
14L3e (9086) Linear Transducer

14L3e (9086) Linear Transducer b Footnote: b) Transducer connector is not immersible.
 Print Cleaning Methods
Print Cleaning Methods
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
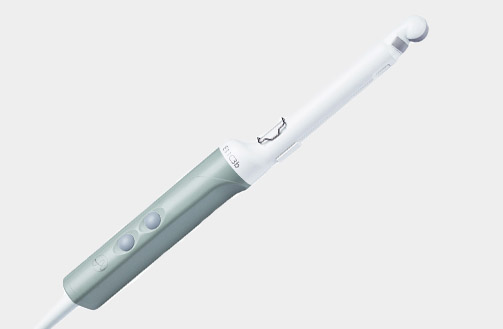
E11C3b (9008) High-resolution biplane Transducer
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
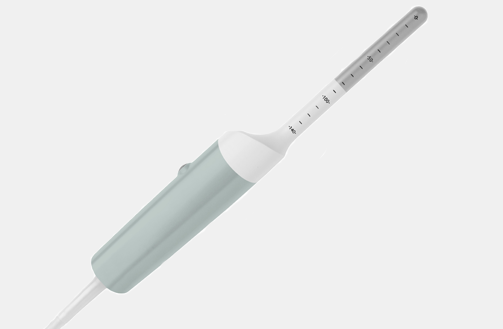
3D X14L4 (9038) Endocavity Transducer
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
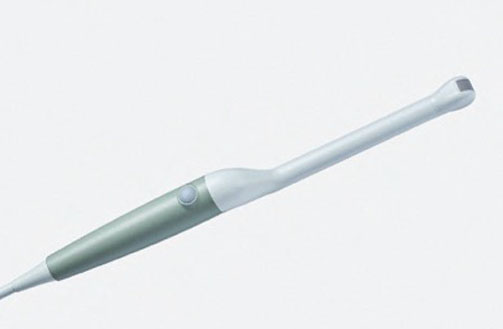
E13C2 (9029) High-res. Broad Bandwidth, Endfire Transducer
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
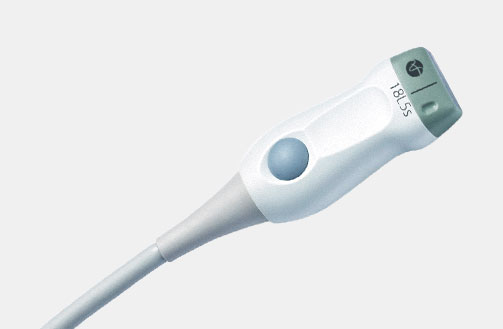
18L5s (9081) Small High-Frequency Linear Transducer
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
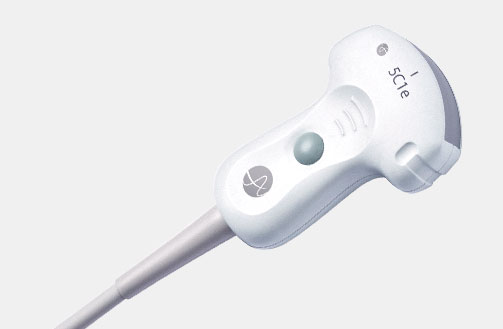
5C1e (9085) Curved Array Transducer

5C1e (9085) Curved Array Transducer b Footnote: b) Transducer connector is not immersible.
 Print Cleaning Methods
Print Cleaning Methods
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
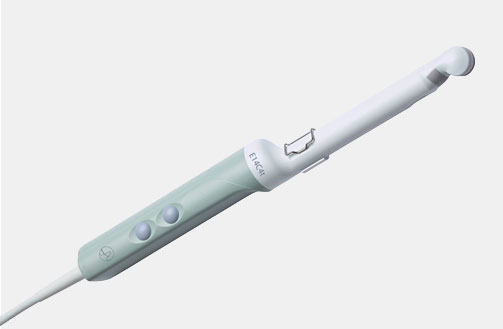
E14C4t (9018) Prostate Triplane Transducer
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
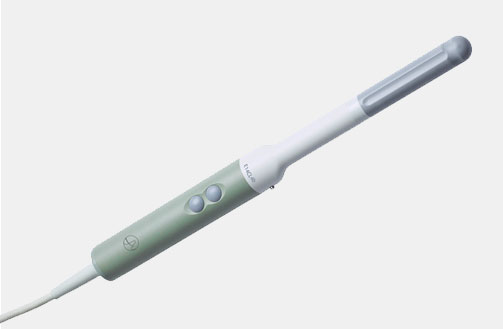
E14CL4b (9048) Endocavity Biplane Transducer
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
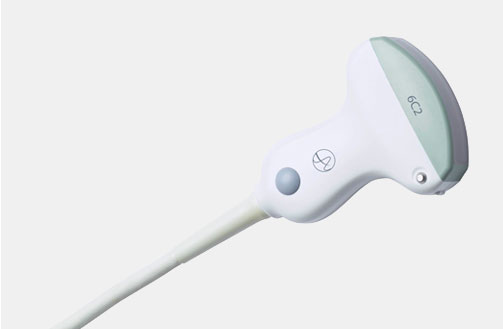
6C2 (9040) Curved Array Transducer
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.

13L4w (9011) Wide Linear Array Transducer
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
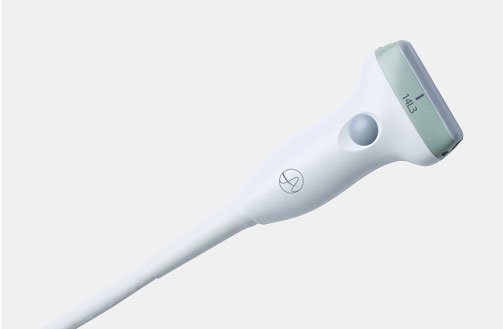
14L3 (9051) Linear Array Transducer
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.

18L5 (9070) High Frequency Linear Transducer
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
N13C5 (9062) Craniotomy Transducer
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
5P1 (9077) Small Footprint Cardiac Transducer
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
E10C4 (9019) Endocavity Transducer
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.

X12C4 (9026) Robotic Drop-In Transducer
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
I14C5I (9015) Intraoperative Transducer
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
I14C5T (9016) T-Shaped Intraoperative Transducer
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
Efficacy validated by BK
Material Compatible a Footnote: a) “Material compatible” indicates that BK Medical has evaluated the device’s material compatibility with the reprocessing method when reprocessed according to the product/system IFU. Efficacy is not covered by this statement.
